| Phase change | Value |
|---|---|
| Evaporation Condensation |
2501 \(kJ kg^{-1}\) |
| Melting Freezing |
334 \(kJ kg^{-1}\) |
| Sublimation Deposition |
2835 \(kJ kg^{-1}\) |
Energy
The ability to do work.
Learning Objectives
- Understand which forms of energy are important for driving the climate system.
- Explain the difference between heat and temperature.
- Describe the mechanisms by which energy is transported.
- Understand how we account for energy and mass conversions in the atmosphere.
iClicker
We’ll try using I clicker today:
- Use the join code: PXPZ
- Or follow this link: https://join.iclicker.com/PXPZ
If these options don’t work, go to:
- student.iclicker.com
- Then search for Atmospheric Environments ## iClicker
We’ll try using I clicker today:
- Use the join code: PXPZ
- Or follow this link: https://join.iclicker.com/PXPZ
If these options don’t work, go to:
- student.iclicker.com
- Then search for Atmospheric Environments
Which forms of energy are important in the atmosphere?

Test Poll
I am able to join and answer:
A - Yes
B - No
Test Poll
I am able to join and answer:
A - Yes
B - No
Forms of energy in the atmosphere
- Radiation: electromagnetic waves (e.g., sunlight)
- Sensible heat: thermal energy we can feel (e.g., warm air, cold ice)
- Latent heat: phase changes of a water (e.g., evaporation, condensation)
- Chemical energy: bonds of atoms (e.g., photosynthesis)
- Kinetic: from motion (e.g., winds)
- Geopotential: position in gravitational field
Heat vs. Temperature
Heat is the thermal energy
- Sum of kinetic and potential energy
Temperature a (relative) measure of thermal energy.
- Average random kinetic energy
- The ability of a body to transfer thermal energy
Heat vs. Temperature
Heat is thermal energy
- Travels from hotter to colder objects
- Can do work
- Often expressed in Joules (J)
- J = 1 N x 1 m
- Newton (N) = 1 kg m s-2
Temperature a (relative) measure of thermal energy.
- Increases/decreases when heat is added/removed
- Cannot do work
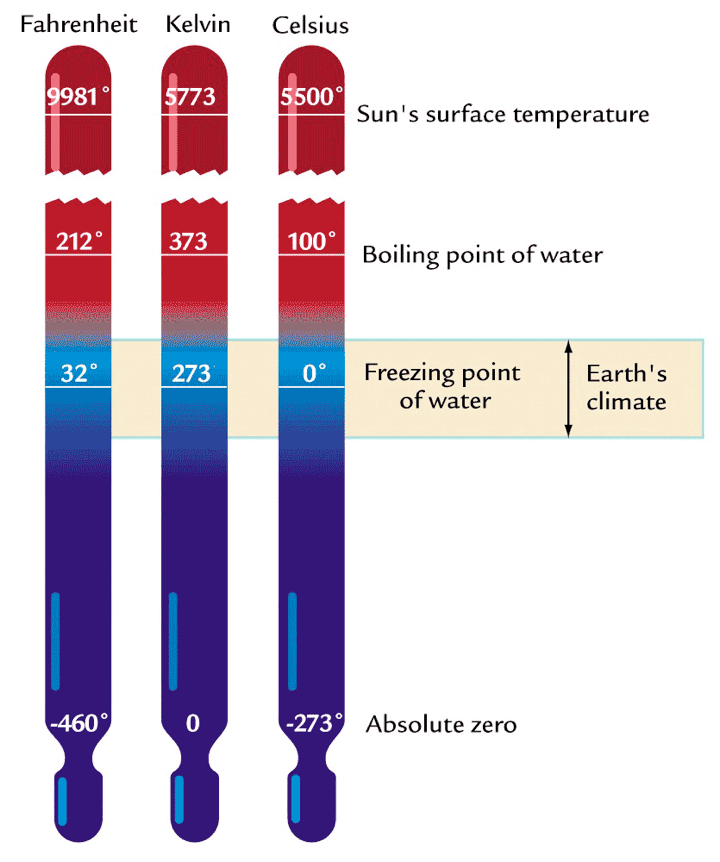
Temperature scales compared
Several scales have been invented:
Fahrenheit, Celsius, Kelvin…
According to the SI system
- We should use Kelvin (K)
- or Celsius (°C)
- For absolute temperatures only
Conversion:
\[ T(K) = T(\deg C) + 273.15 \qquad(1)\]
Three States of Water

States of Water (iClicker)
Which of the following are not states of water? ## States of Water (iClicker)
Which of the following are not states of water?
- A: Ice
- B: Liquid
- C: Vapor (gas)
- D: Air
- A: Ice
- B: Liquid
- C: Vapor (gas)
- D: Air
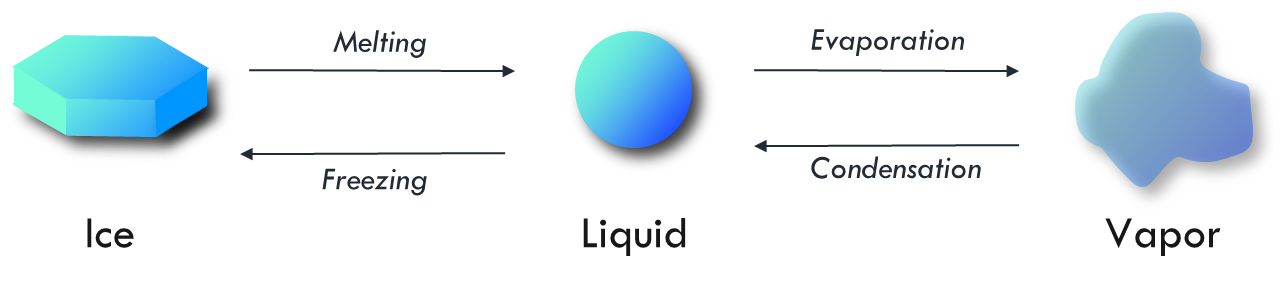
State changes of water

State changes of water
Latent Heat

Latent Heat

Latent Heat
The energy (\(kJ\: kg^{-1}\)) required for water to change states varies with temperature.
Latent heat of vaporization
Conversion from latent to sensible heat in a storm cloud is equivalent to the energy released from a small nuclear bomb.
- Based on the amount of latent heat picked up at the surface through evaporation
- Released as water vapor condenses back into liquid water or freezes into ice.

Why spray liquid water on a tree?
Its seems counter-intuitive, but fine mist irrigation by sprinklers is used to reduce frost damage.

Why spray liquid water on a tree?
Latent heat of fusion:
- Sprayed liquid water releases latent heat of fusion as it becomes ice
- Prevents a damaging drop in temperature of almonds

Energy transfer in the atmosphere
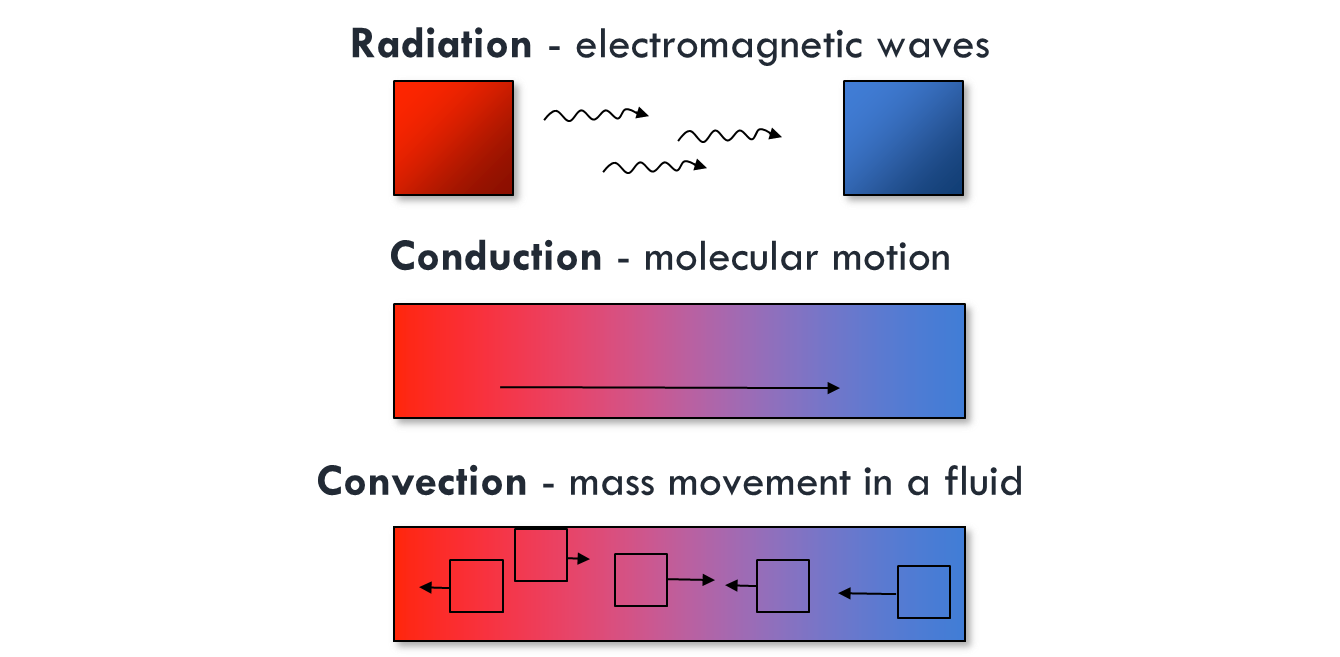
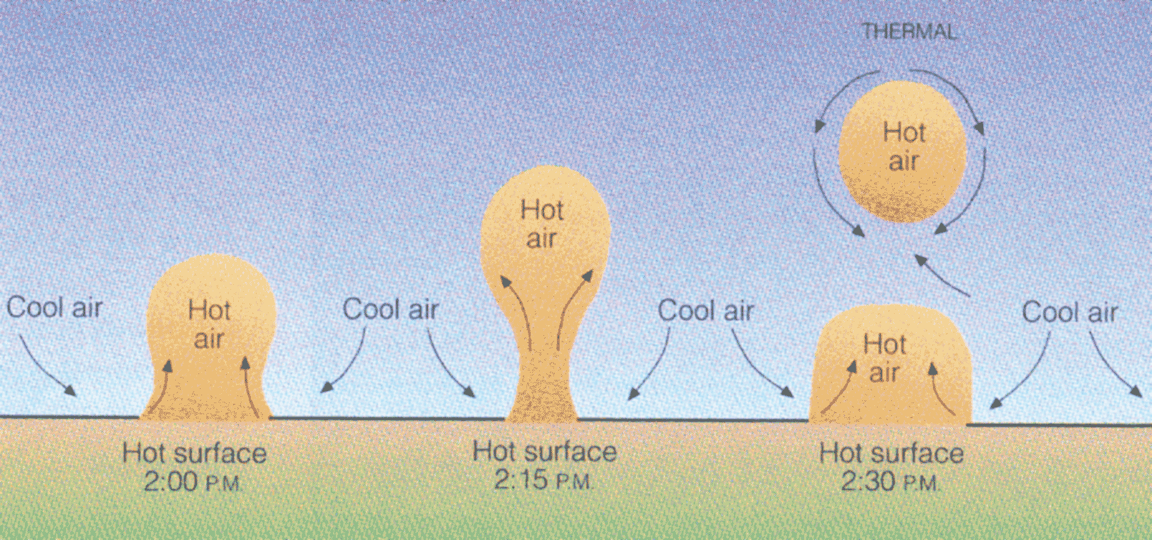
Convection
There are free and forced types of convection.
- Forced convection would be air movement caused by a fan or the wind.
Fluxes and flux densities
Heat
- Aka. Energy
- J (Joules)
Heat Flux
- Flow rate of energy
- Aka. Power
- W = J s-1
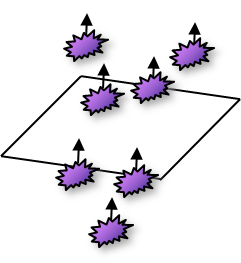
Heat Flux Density
- Net transfer of energy
- Flow rate of energy per unit area
- W m-2 = J s-1 m-2
Heat Flux (iClicker)
Heat always travels from:
- A: Hotter to colder
- B: Colder to hotter
- C: Either A or B
Fluxes and flux densities
Generally speaking: flux densities can be positive or negative.
- Sum of positive and negative fluxes
- The sign will depend on your reference point.
- Heat will always go from hotter to colder object
- But many fluxes are bi-directional.
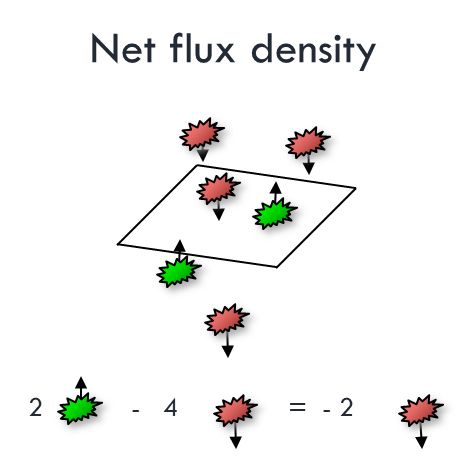
Net Radiation
- Incoming radiation (sunlight)
- Minus reflected & emitted radiation

Net Ecosystem Exchange (of CO2)
- Carbon uptake (photosynthesis)
- Minus carbon emission (respiration)
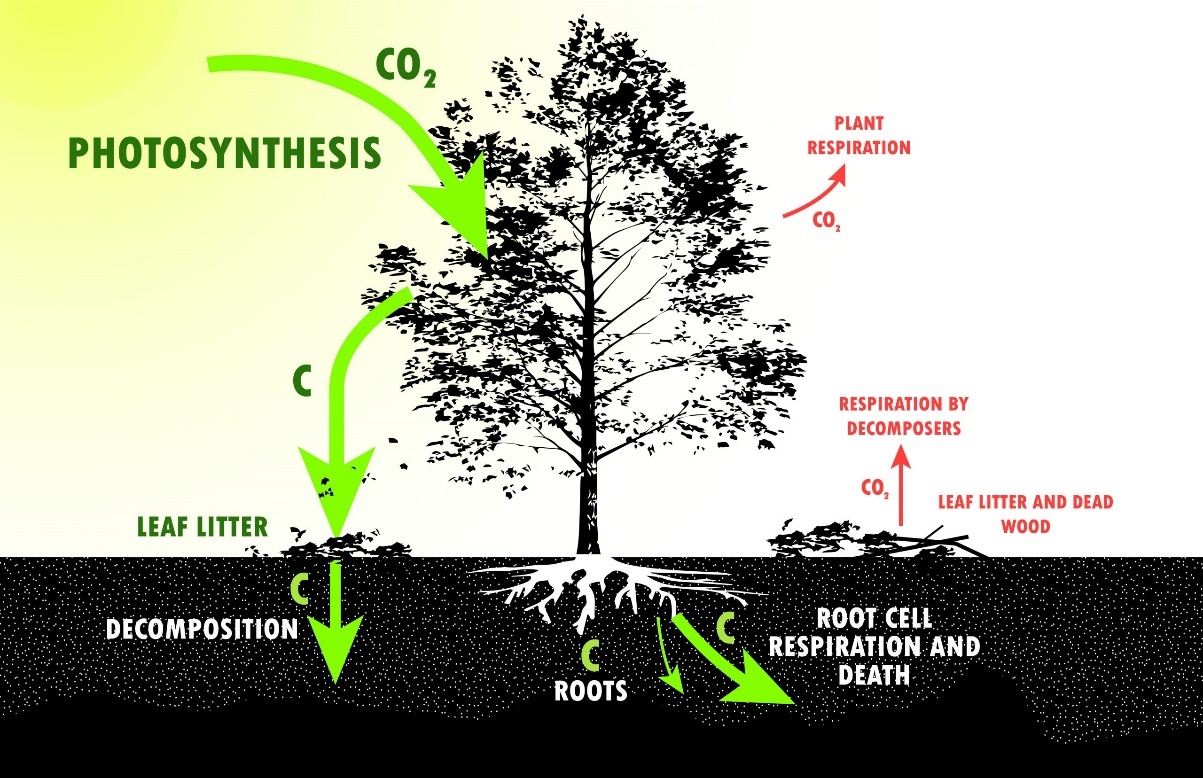
Conservation of energy and mass
One of the most powerful laws used in analyzing organism-environment interaction is this Law of Conservation.
- Neither mass nor energy can be created or destroyed by any ordinary means.
- The application is similar to reconciling your checking account i.e. you can construct a budget or balance to account for all inflows and outflows of heat and mass.
Conservation of energy and mass
Energy is continually being converted from one form to another
- None is lost.

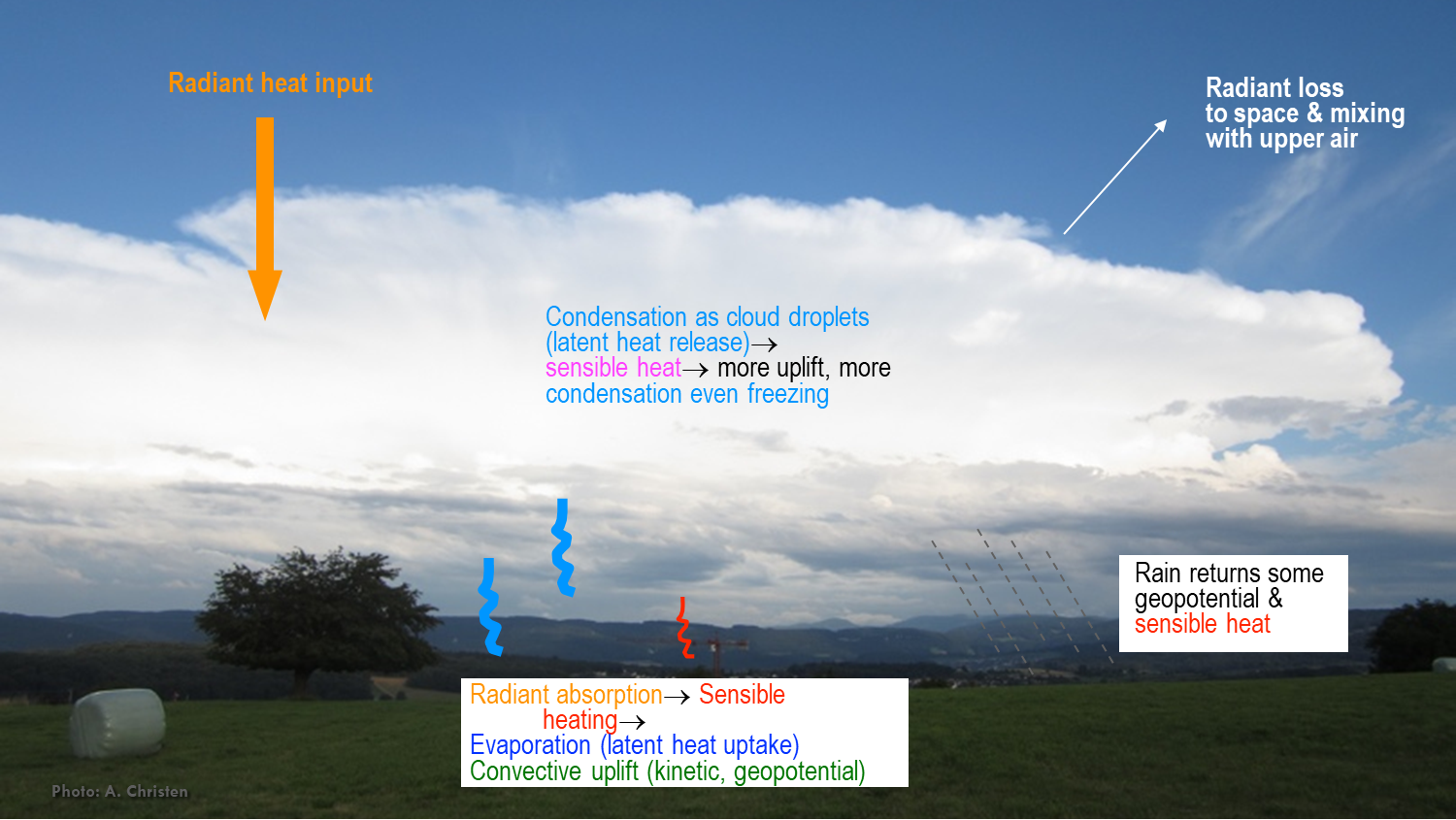
Energy conservation
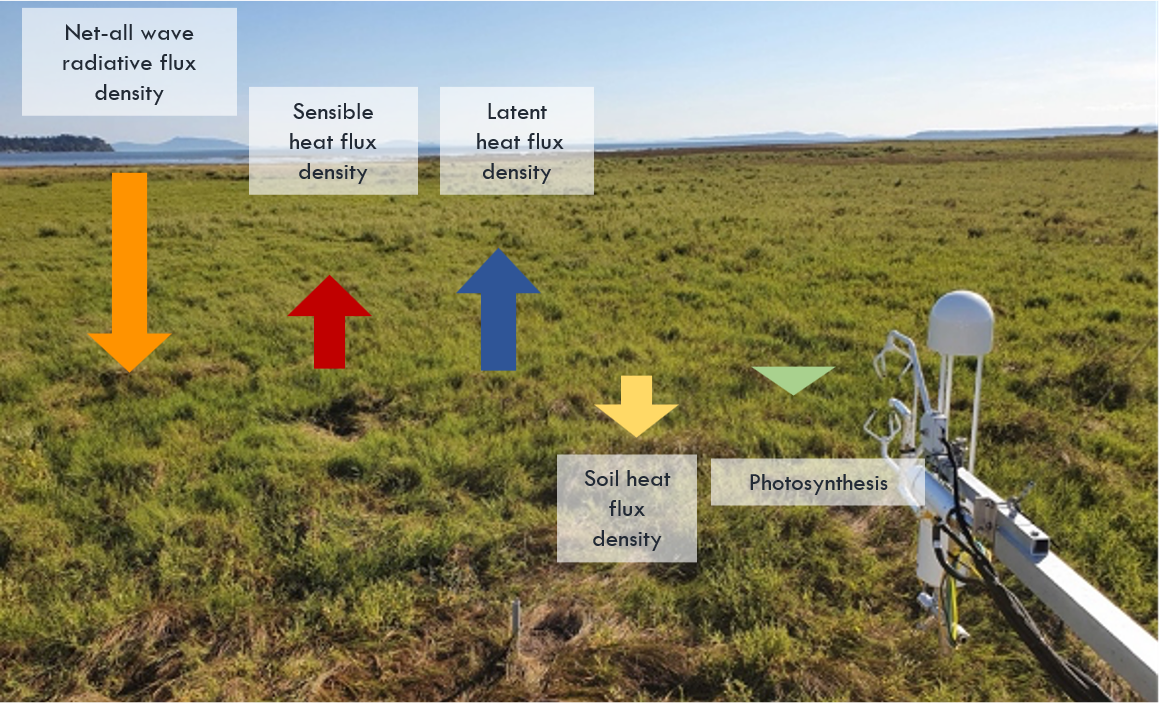
Energy balance of a vegetated surface
Summary
- Difference between heat and temperature
- Heat does work - temperature does not
- What is latent heat?
- Energy associated with phase change (of water)
- Flux vs. flux density
- Flux density is energy transport per unit area per unit time (W m-2)
Summary
- Energy and mass transfer mechanisms
- Radiation, conduction, and convection
- Understand the concept of energy and mass balances and their connectivity
- Neither mass nor energy can be created or destroyed by any ordinary means, just converted from one form to another.