Atmospheric Humidity
Quantifying water vapor content in the atmosphere
Atmospheric Humidity

An Assmann Psycrhometer can be used to measure the wet bulb temperature and calculate atmospheric humidity
Learning objectives
- Describe the physical and biological significance of atmospheric humidity
- Define vapor density (or absolute humidity)
- Be able to convert between vapor density and vapor pressure
- Understand the relationship of saturation vapor density to temperature
- Define and know how to calculate relative humidity
- Know other important measures of humidity - vapor density deficit, vapor pressure deficit, dew point temperature & wet bulb depression
- Give examples of sensors that can measure humidity
Atmospheric Humidity
Water vapor makes up small part of atmosphere, but has important physical and biological significance because:
- It’s a major greenhouse gas
- Plays a major role in energy transport globally and locally
- Affects animal comfort, plant disease, fire weather, metal corrosion, etc.
| Gas | Proportion |
|---|---|
| Nitrogen (N2) | 78.08% |
| Oxygen (O2) | 20.95% |
| Argon (Ar) | 0.93% |
| Carbon Dioxide (CO2) | 0.04% |
| Misc. Trace Gasses | <0.01% |
| Water Vapor (H2O) | 0-3% |
Atmospheric Humidity
- The heat released by condensing 1 g of water is enough to raise 1 kg of air (0.8 m3) by 2.5 °C
- Due to water’s very high heat of vaporization, 2450 J g-1
- Freezing water releases another 330 J g-1
- We feel cold after coming out of a swimming pool because the heat used for evaporation of the water comes from our skin
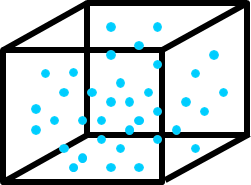
Vapor Density
Vapor density (\(\rho_v\)) is the mass of water vapor per unit volume of air (g m-3); it is the fundamental measure of humidity.
- The ‘driving force’ for evaporation is the \(\rho_v\) gradient between an evaporating surface and the surrounding air.
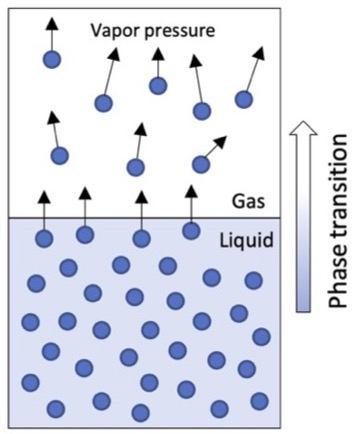
Vapor Pressure
Vapor pressure (\(P_v\)) is the partial pressure (aka force) exerted by water vapor in a parcel of air.
A slightly more abstract measure of humidity.
Mean sea level air pressure is 101.325 kPa
\(P_v \leq 5 kPa\)
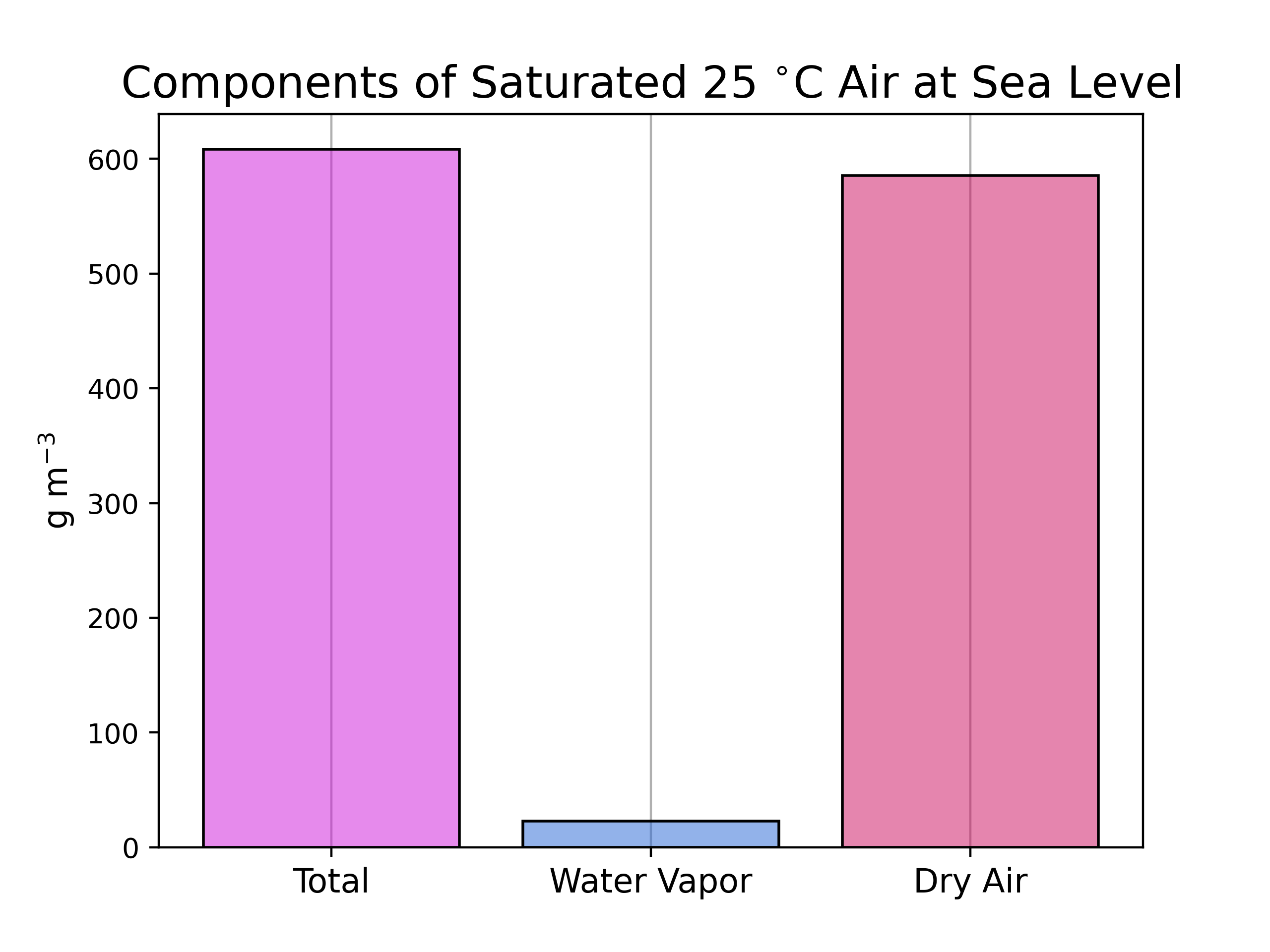
Mixing Ratio
Water vapor content can also be expressed as a mixing ratio, which gives the abundance of one component of a mixture (H2O) relative to all other components (“dry air”).
\(r_{H2O} = \frac{m_{H2O}}{m_{dry}}\) \(m_{dry} = m_{total} - m_{H2O}\)
- Usually parts per thousand for water vapor
- g H2O per kg dry air
- In this example:
\(m_{dry} = 608.5 g - 23 g = 0.5855 kg\)
\(r_{H2O} = \frac{23 g}{0.5855 kg} = 39.28 \frac{g}{kg}\)
Ideal Gas Law
Describes the state of a gas as a function of pressure (\(P\)), volume (\(V\)), temperature (\(T\)) in Kelvin, the amount of the gas (\(n\)) in moles; and the ideal gas constant (\(R\) = 8.314 x 10 \(^{-3}\) kPa m \(^3\) g \(^{-1}\) K \(^{-1}\)).
\[ PV=nRT \qquad(1)\]
- \(n = \frac{m}{M}\), where \(m\) is the mass of the substance and \(M\) is the Molar mass of the substance.
- Can be applied to a mixture of gasses (e.g., dry air) or a single gas (e.g., water vapor)
Ideal Gas Law
The molar mass of water is 18.015 g mol \(^{-1}\). Given this, we can redefine Equation 1 in terms of \(\rho_v\) and \(P_v\) as Equation 2.
\[ \rho_v = \frac{P_vM}{RT} \qquad(2)\]
- This allows us to convert between \(\rho_v\) and \(P_v\)
- Knowing one, allows easy calculation of the other
Saturation
The ability of air to hold water vapor increases exponentially with temperature.
- We add a “\(^*\)” to indicate saturation:
- Saturation vapor density \(\rho_v^*\)
- Saturation vapor pressure \(P_v^*\)
Laten Heat
Determining \(\rho_v^*\) or \(P_v^*\) for a given \(T\) requires integrating the Clausius–Clapeyron equation.
- The latent heat of vaporization \(L\) is also varies as a function of \(T\)
\[ \frac{d P}{d T} = \frac{L P}{R T^2} \qquad(3)\]
Saturation
Luckily, some experimentally based empirical relationships have been developed for typical conditions on Earth’s surface.
- It turns out temperature (\(T\)) and pressure (\(P\)) changes have an equal and opposite effect on saturation pressure \(P_v^*\)
- We can calculate \(P_v^*\) to \(\pm\) 0.1% for typical temperature conditions using the empirically derived Buck Equation:
\[ P_v^*=0.61121e^{(18.678-\frac{T}{234.5})(\frac{T}{257.14+T})} \qquad(4)\]
Saturation
Partial Saturation
Typically, ambient air is not saturated. An air parcel such as the one the figure can be brought to saturation by:
- Adding moisture to the air
- Cooling the air
Dewpoint temperature (Td)
The temperature down to which the air must be cooled isobarically (without changing air pressure) for it to become saturated.
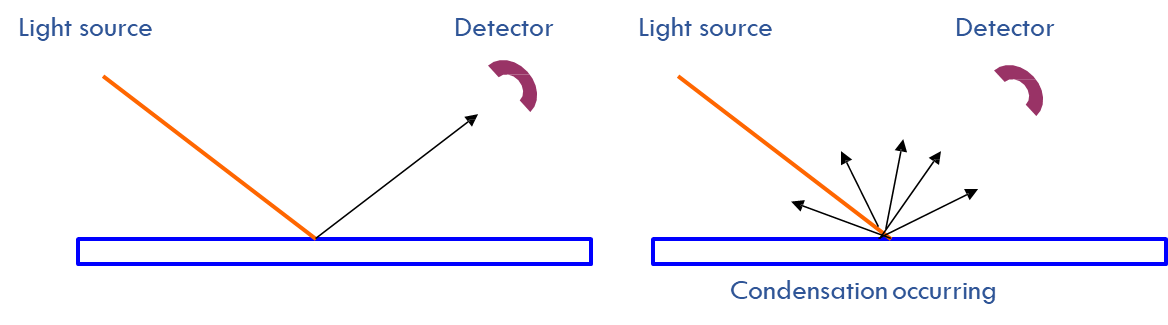
Can be measured with a silver coated cooled mirror. \(T_d\) is the temperature of the mirror when water vapor condenses on it.
Dewpoint Calculation (iClicker)
You can determine \(T_d\) from \(\rho_v\) or \(P_v\) using the relationship between \(T\) and \(\rho_v^*\) or \(P_v^*\)
If \(T\) = 20 °C and \(P_v\) = 1.7 kPa, what is \(T_d\)?
- A 20 °C
- B 15 °C
Condensation
If an air parcel to cools below \(T_d\), condensation will* occur.
- Condensation releases heat which warms the surrounding environment
- Cloud formation is an important avenue of energy exchange
- Condensation nuclei are required to initiate condensation.
- In the absence nuclei, which can occur in the upper atmosphere, air will become supersaturated
- Earth’s surface is a nuclei, so supersaturation doesn’t occur near the surface.
Relative Humidity
Humidity can be expressed as a fraction or % of the maximum (saturation) water vapor the air can hold at that temperature:
\[ RH = \frac{\rho_v}{\rho_v^*} \qquad(5)\]
- RH depends on T because \(\rho_v^*\) is a function of T
Relative Humidity and Temperature
The variation in RH during the day is largely determined by the variation in temperature.
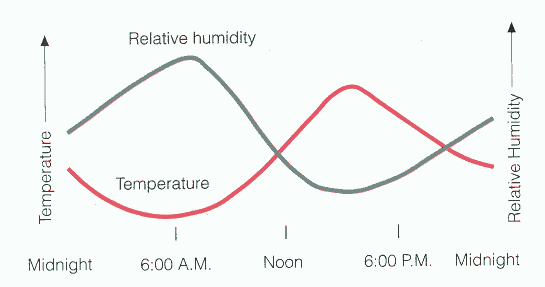
Importance of RH
- Determines the water content of porous media e.g., wood, soil, clothing, which has been allowed to equilibrate with the air.
- Low RH (< 30%) contributes to high risk of forest fire
- Metal corrosion is directly related to RH.
- Many plant diseases are strongly affected by RH.
Relative Humidity (iClicker)
In the classroom, T = 21°C and \(\rho_v\) = 9 g m-3. What is the RH?
- A 5%
- B 100%
- C 25%
- D 50%
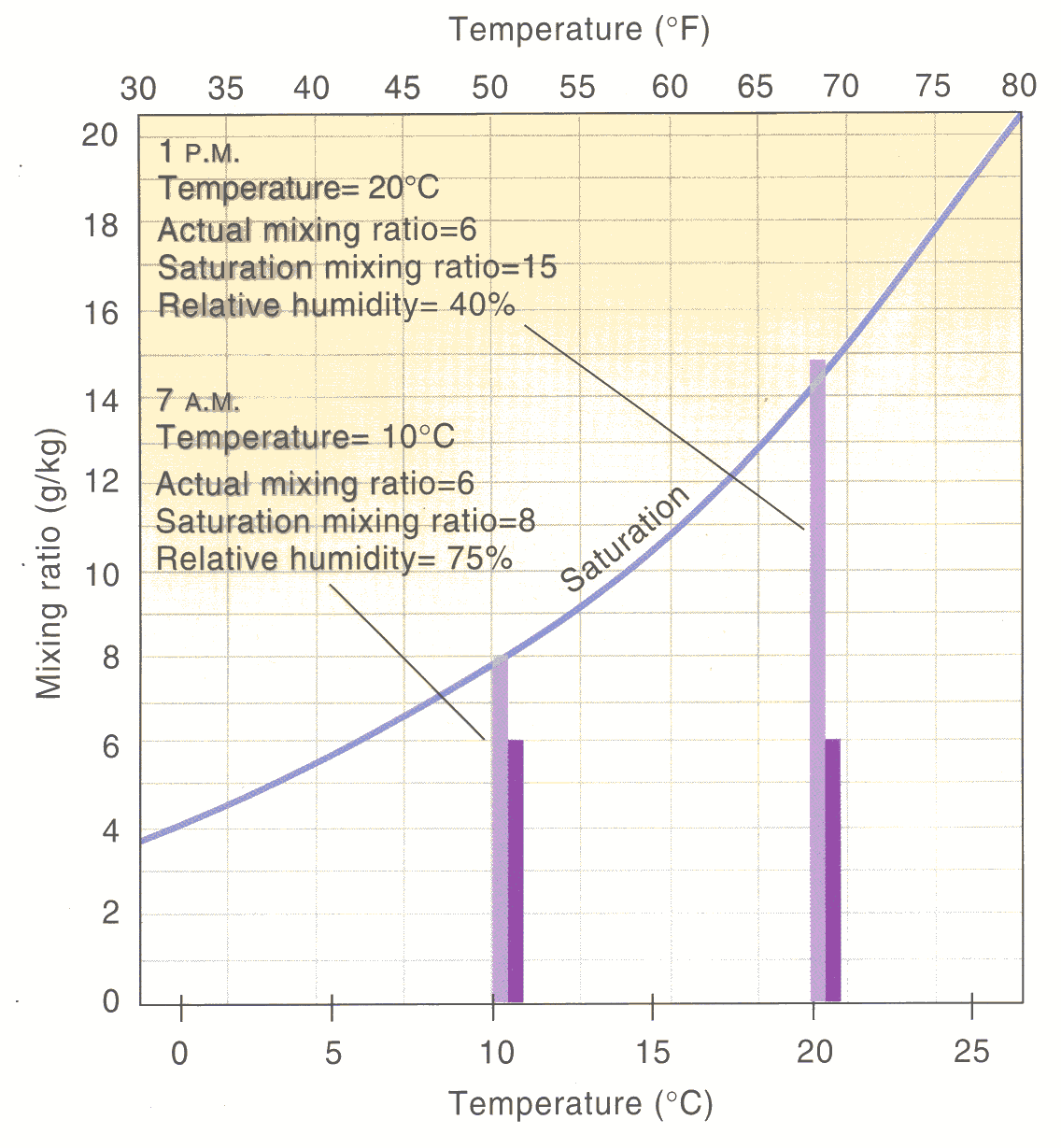
Relative Humidity example
- In the classroom, \(T\) = 21 °C and \(\rho_v\) = 9 g m-3. What is the RH?
- First determine \(\rho_v^*\) using the graph. It is \(\approx\) 18 g m-3.
- Then RH = \(\rho_v\) / \(\rho_v^*\) = (9/18) x 100% = 50%.
Vapor Density Deficit (VDD)
The increase in \(\rho_v\) necessary to bring the air to saturation It is expressed as:
\[ VDD = \rho_v^* - \rho_v \qquad(6)\]
- RH doesn’t determine the rate of evaporation or condensation
- The difference between vapor density at the exchange surface and the air determines it!
VDD (iClicker)
In the classroom, T = 21°C and \(\rho_v\) = 9 g m-3. We know that \(\rho_v^* \approx\) 18 g m-3. So What is the VDD?
\(VDD = rho_v^* - rho_v =\) 9 g m-3
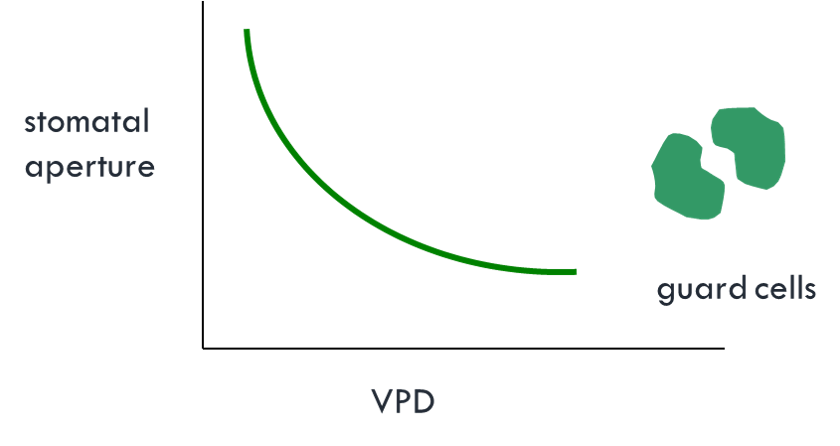
Vapor Pressure Deficit (VPD)
This variable is very similar to VDD
\[ VPD = P_v^* - P_v \qquad(7)\]
- Helpful to understand the response of the stomata of leaves to the dryness of the air
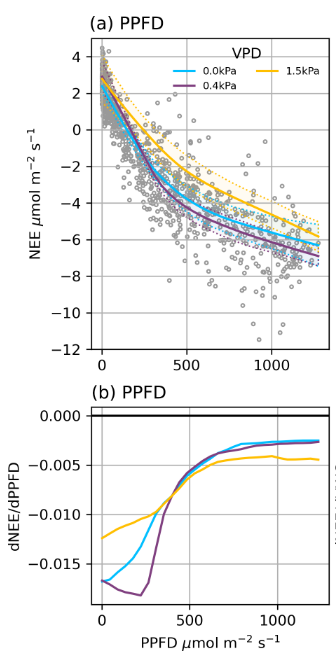
Vapor Pressure Deficit (VPD)
Rates of photosynthesis are influenced by VPD.
- With increasing VPD, photosynthesis first becomes more efficient as the pressure gradient aids gas transfer between the leaves and the atmosphere
- Then it has a strong limiting effect as plants close their stomata to avoid excess water loss
Measuring Humidity
- RH has been measured by different methods: Leonardo de Vinci used the weight of a ball of wool.
- de Saussure invented the hair hygrometer (2.5% change in length for 0 to 100% RH).
- At fire weather stations, the weight of small sticks of wood has been used.
- More recently changes in electrical conductivity resulting from adsorption of water vapor by a thin polymer film coated on small sensing chips has proven to be very effective.
- These are commonly used in climate stations.
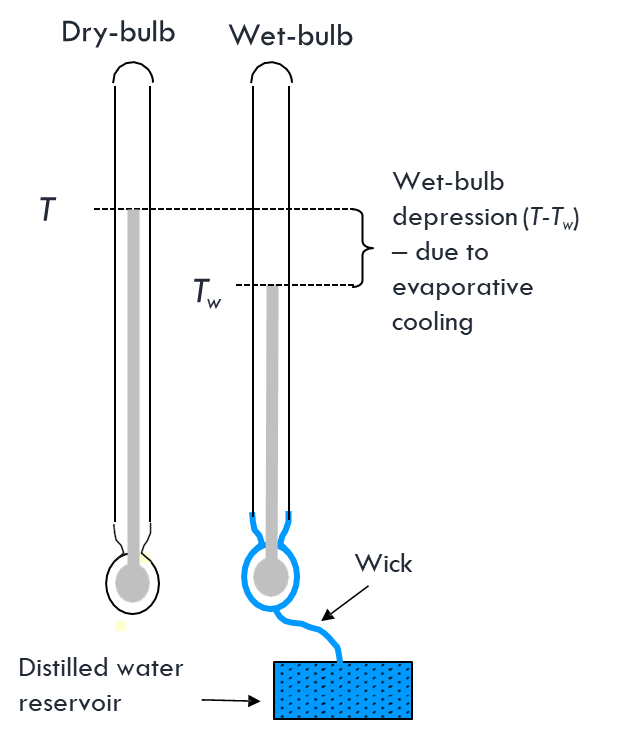
Measuring RH


Psychrometry
- The size of the depression is a measure of the rate of removal of latent heat
- Which is due to the dryness of the ambient air relative to saturation at the temperature of the evaporating surface (the wet-bulb)
Infrared gas analyzer (IRGA)
Fast-response IRGA using the absorption of near-infrared radiation by water vapor (or CO2) as the means of sensing humidity.
- Used in the eddy-covariance method of measuring fluxes of water vapor
- we’ll discuss later.

Take home points
- Makes up small part of atmosphere but is the main greenhouse gas
- Large latent heat of vaporization (conversion of liquid to vapor)
- Relationship of vapor density to vapor pressure by ideal gas law
- Saturation vapor density (ability of air to store water vapor) (v *) is exponentially related to temperature
- RH (v/v ) and VDD (v - v) are very sensitive to temperature
- vapor density difference determines rate of H2O vapor transfer
- RH determines equilibrium water content of porous materials such as fire fuels & affects animal comfort, plant disease & metal corrosion
- Leaf stomata close as VDD or VPD increases