| Surface | X epsilon |
|---|---|
| Soil | 0.90 – 0.98 |
| Grass | 0.90 – 0.95 |
| Crops | 0.90 – 0.99 |
| Forests | 0.97 – 0.99 |
| Water | 0.92 – 0.97 |
Radiation Laws
The earth climate and ecosystems are driven by radiation emitted by the sun.
Learning Objectives
- Understand exactly what is radiation and why it is relevant in biometeorology & climate science.
- Describe the quantity and quality of radiative energy.
- Know which physical laws can be used to predict quantity and characteristics of radiation emitted.
Radiation Laws
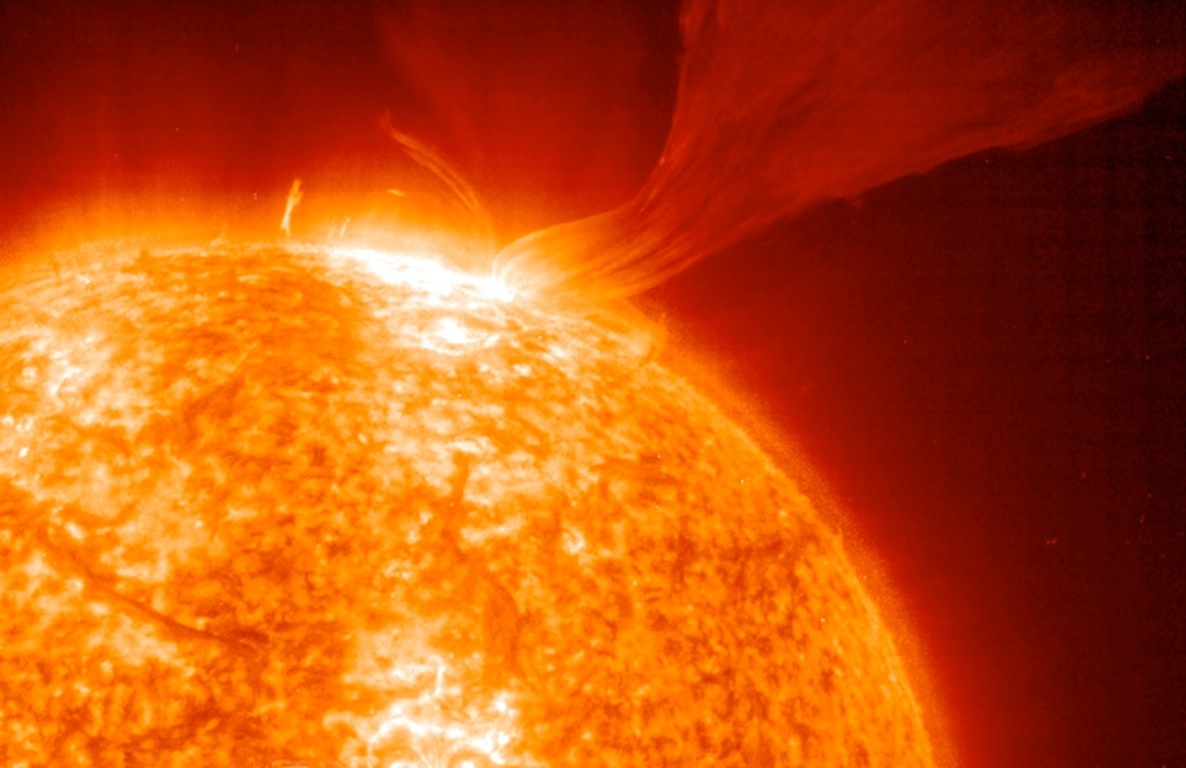

Radiation is emitted from all matter
GEOB 200 / APBI 244 students emit radiation!

Characteristics of Radiation
It is the emission of energy as electromagnetic waves or as moving particles.
- Can travel in a vacuum (space).
- Travels in straight lines at up to \(c = 3 * 10^{8} m s^{-1}\)
- Takes 8 min 20 s to travel from the Sun to the Earth.
- Travels in straight lines at up to \(c = 3 * 10^{8} m s^{-1}\)
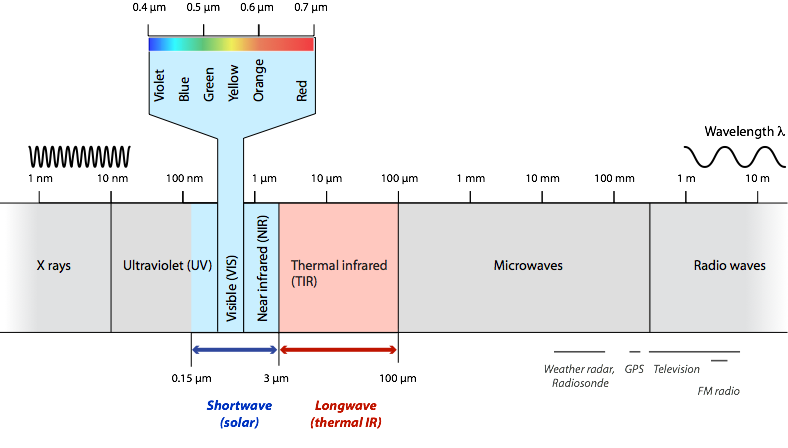
The electromagnetic spectrum
Radiant flux density
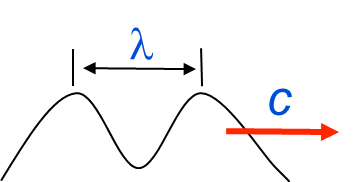
Wave-like
- Wavelength \(\lambda\) (m) & frequency \(ν=\frac{c}{\lambda}\)
- c is the speed of light.
\[ v=\frac{c}{\lambda} \qquad(1)\]
Particle-like
- Energy emitted in discrete packets (Photons)
- Energy of a photon
- Planck’s constant: \(h = 6.63 * 10^{-34} J s\)
\[ e = hv \qquad(2)\]
Energy content of a photon (iClicker)
What is the energy content (\(e\)) of one photon of yellow light (\({\lambda} = 0.55 \mu m\))?
\(v=\frac{c}{\lambda}\)
\(e=hv\)
- Note:
- \(c = 3 * 10^{8} m s^{-1}\)
- \(h = 6.63 * 10^{-34} J s\)
- \(1 \mu m = 10^{-6} m\)
- A: \(3.6 x 10^{-19} J\)
- B: \(4.1 x 10^{45} W\)
- C: \(0.1 x 10^{-3} s^{-1}\)
- D: \(10.1 x 10^{5} m^{2}\)
Energy content of a photon (iClicker)
What is the energy content (\(e\)) of one photon of yellow light (\({\lambda} = 0.55 \mu m\))?
\(v=\frac{c}{\lambda}\)
\(e=hv\)
- Note:
- \(c = 3 * 10^{8} m s^{-1}\)
- \(h = 6.63 * 10^{-34} J s\)
- \(1 \mu m = 10^{-6} m\)
A: \(3.6 x 10^{-19} J\)
\(v = \frac{3 * 10^{8} m s^{-1}}{6.63 * 10-34 J s}\)
\(v = 5.5 * 10^{14} s^{-1}\)
\(e = 6.63 * 10^{-34} J s * 5.5 * 10^{14} s^{-1}\)
\(e = 3.6 * 10^{-19} J\)
Why does it mater?
The calculation is important for photosynthesis. Plants respond to the number of photons they absorb rather than simply to the total energy absorbed.
- Plants can only use photons in the visible range of the spectrum
- This region is also called photosynthetically active radiation (PAR)

Radiation vs. Temperature
Wien’s displacement law
The wavelength of maximum emission (\(\lambda_{max}\)) from an object is inversely proportional to the temperature T (in K) of its surface:
\[ \lambda_{max} = \frac{b}{T} \qquad(3)\]
- A general approximation for a ‘blackbody’
- b ≈ 2898 μm K

Wien’s displacement law
Can be used to determine T of an object without having to measure the flux density of emitted radiation.
- As \(T \uparrow\) >> \(λ_{max} \downarrow\)
- A hot iron:
- Dull red > bright red > orange > yellow > white
- Wavelength of red is longer than that of yellow

Short-wave vs. Long-wave
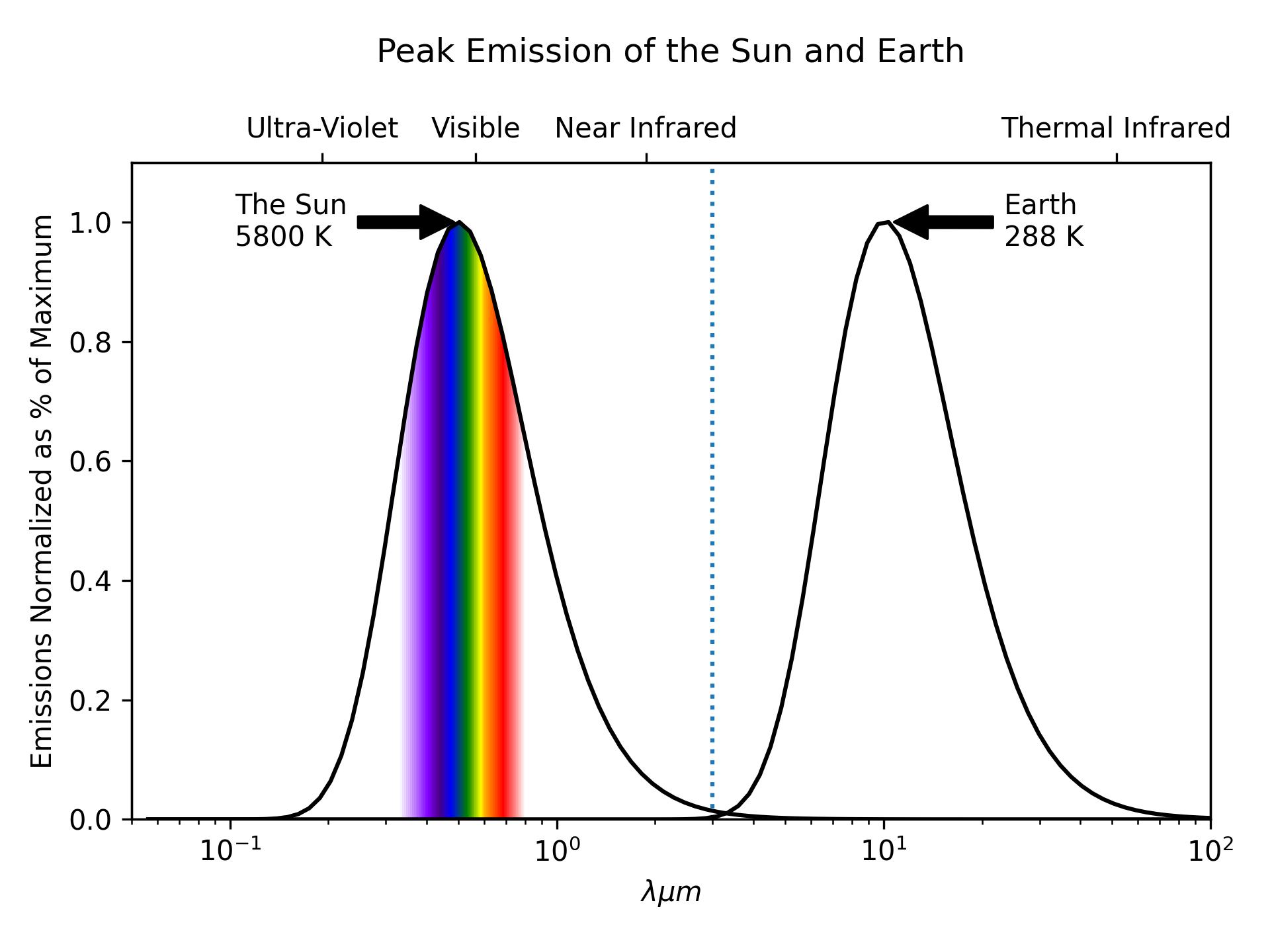
Short-wave vs. Long-wave
The exact boundary will vary from source to source; \(\lambda = 3 \mu m\) will be where we draw the distinction.
- Short-wave:
- Higher energy radiation from the sun
- Long-wave:
- Lower energy thermal radiation

Your peak emissions (iClicker)
The human body temperature is typically around 37 \(^{\circ}\) C, which is 310.15 K. What would the wavelength (\(\lambda\)) of a typical human’s peak emissions be (in \(\mu m\)) following Wein’s Law?
- \(\lambda_{max} = \frac{b}{T}\)
- \(b ≈ 2898 \mu m K\)
\(\lambda_{max} = \frac{2898 \mu m K}{310.15 K} = 9.34 \mu m\)
Stefan-Boltzmann law: blackbody
The total energy emitted by a blackbody \(Eb\) (emittance) is proportional to fourth power of its absolute Temperature:
\[ E_b = \sigma_b T^4 \qquad(4)\]
\(\sigma_b = 5.67 * 10^{-8} W m^{-2}K^{-1}\)
- \(\sigma_b\) is easy to remember
- “5, 6, 7, 8”
Grey bodies
Emission from natural objects is given by adjusting the Stefan-Boltzman Law to account for surface emissivity (\(\epsilon\)).
- Emissivity is the ratio of the actual emission to that of a blackbody (\(\epsilon\) = 1.0).
\[ E_g = \epsilon\sigma_b T^4 \qquad(5)\]
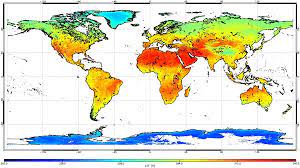
Stefan-Boltzman Applications
The the basis of remote sensing in thermal satellite sensors and infrared thermometer use it to measure the surface temperature of objects

Total Emissions
Building on Wein and Stefan-Boltzman, we can use Plank’s law to calculate the total emissions from a body across the electromagnetic spectrum.
\[ E = \frac{2*h*c}{\lambda^{5}}*\frac{1}{e^{\frac{h*c}{\lambda*\sigma_b*T}}-1} \qquad(6)\]
- \(\sigma_b = 5.67 * 10^{-8} W m^{-2}K^{-1}\)
- \(c = 3 * 10^{8} m s^{-1}\)
- \(h = 6.63 * 10^{-34} J s\)
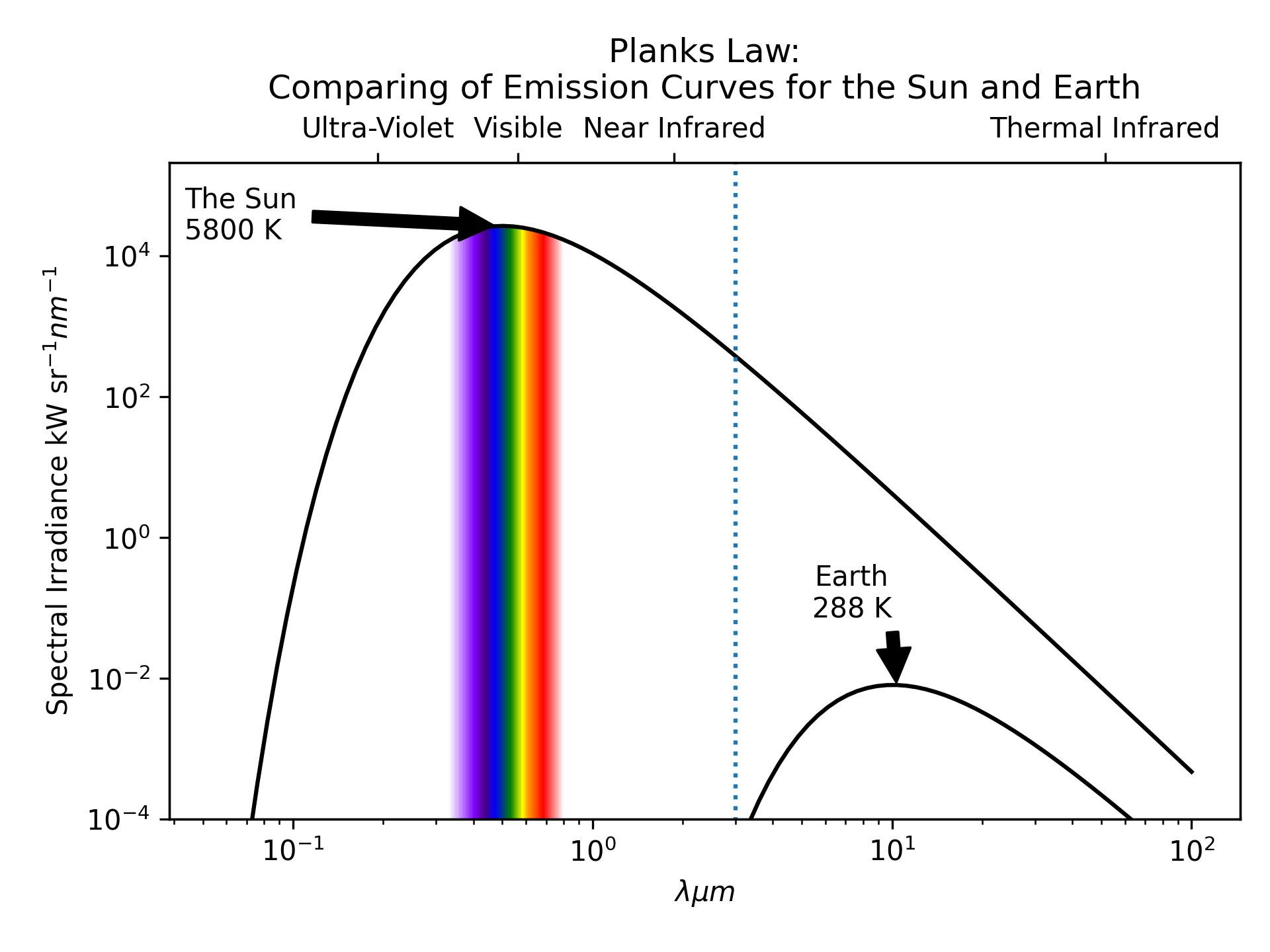
Total Emissions
Note the log scale. At their respective peaks the Sun’s emissions are \(10^{6}\) greater than the Earth’s.
- The Sun emits more radiation than the Earth
- Across all wave lengths.

Radiation Balance
Radiation (of wavelength \(\lambda\)) can interact with an object in three ways:
- Absorption \(a_{\lambda}\)
- Transmission \(\tau_{\lambda}\)
- Reflection \(\alpha_{\lambda}\)
\[ a_\lambda + \tau_\lambda + \alpha_\lambda = 1 \qquad(7)\]
- These terms vary by object and by \(\lambda\), but all radiation reaching an object must be absorbed, transmitted, or reflected.
Absorption Spectra
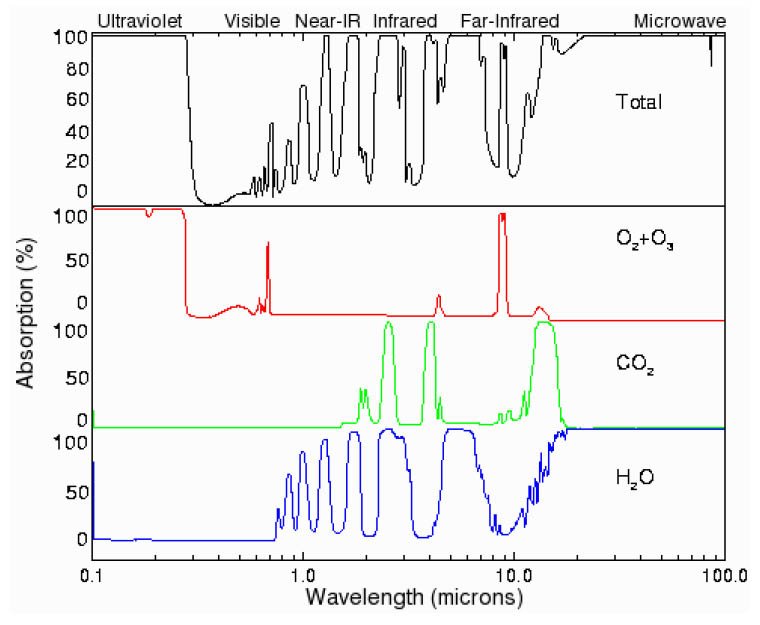
Absorption Spectra of Gasses in the Atmosphere
Reflection and Transmission
Whatever isn’t absorbed, must be either reflected, or transmitted.
- Liquid water transmits visible light well
- It absorbs very little visible radiation
- And it reflects very little too
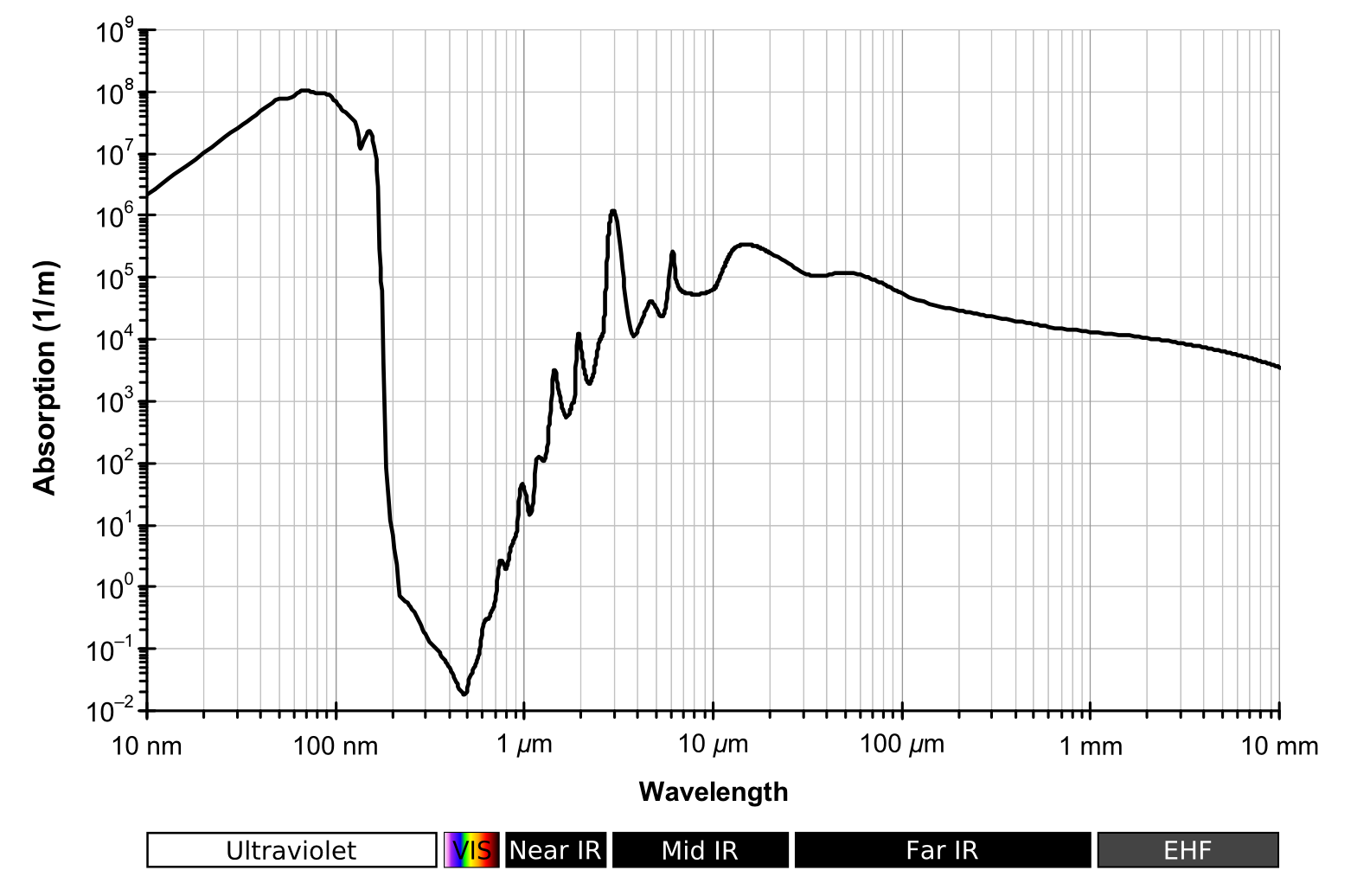
Accounting for reflectivity
Assuming the object isn’t transmissive (\(\tau \approx 0\)), which is the case for large objects like the earth across all but the longest wavelengths: \(a_{\lambda} + \alpha_{\lambda} = 1\)
- We can extend Stefan-Boltzman to account for reflectivity.
\[ E_g = \epsilon\sigma_b T^4 + (1-\epsilon) * (SW\downarrow+LW\downarrow) \qquad(8)\]
Net Radiation
Given this understanding, we can define Net Radiation (\(R_n\)) as the sum of all incoming (\(\downarrow\)) and outgoing(\(\uparrow\)) radiation components. We will specifically distinguish between short-wave (SW) and long-wave(LW) radiation.
\[ R_n = (SW \downarrow - SW \uparrow) + (LW \downarrow - LW \uparrow) \qquad(9)\]
Take home points
- What is the difference between long-wave and short-wave radiation?
- Know the the basic radiation laws: Wien’s Law, Stefan Boltzmann Law, and Plank’s law
- These are idealized for black bodies, but can be modified.
- Radiation can be: absorbed, transmitted, or reflected.